Tantalum and its Alloys
Tantalum and its Alloys
Material Properties and Applications
Brandon DeCicco, Geoffrey Smith, Matt Osborne and Aamir Abid
Tantalum is a refractory blue-grey metal originally discovered in 1802 by Anders Ekeburg from Sweden. It was first commercially used in dental tools and incandescent light bulb filaments due to its chemical inertness and high-melting point1.
As early as 1957, tantalum was recognized for its corrosion resistance in harsh environments where most other metals are not suitable2. Tantalum’s corrosion resistance has been described as similar to glass; however, tantalum is also ductile and has strength similar to steel. Tantalum gets its corrosion resistance from the formation of a passive oxide layer as it initially reacts with oxygen on the surface. The oxide layer is stable at temperatures below 260°C. Tantalum has improved corrosion resistance compared to most typical corrosion resistant metals used in industry such as titanium, zirconium, niobium and nickel alloys. In strong hot acids such as HCl and H2 SO4, tantalum’s corrosion resistance is equivalent or superior to these metals.
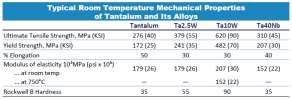
In addition to the corrosion resistance properties, tantalum has excellent physical properties. Like many pure transition metals, tantalum has good ductility and can be worked to form a variety of mill products such as wire, sheet, rod and bar. Typical elongation properties are between 30% – 50% and about twice that of tungsten and some tungsten alloys. Tantalum also has similar elongation compared to titanium and twice the elongation of common titanium alloys such as Ti-6Al-4V3 . In order to further improve strength, tantalum is alloyed with tungsten (Ta2.5W and Ta10W) and these alloys have strength and workability similar to or greater than that of steel. Tantalum alloys are easily worked into a variety of parts such as tubing, thermowells, heat exchangers, industrial chemical apparatus, missile and aircraft parts and surgical instruments. Tantalum can also be alloyed with other elements such as niobium and nickel based super alloys to increase its strength and temperature resistance. Adding niobium to tantalum, reduces weight and saves cost while maintaining good corrosion resistance.
Currently, the majority of tantalum produced is used in the electronics industry for capacitors. Tantalum powders are pressed and sintered to form porous anodes. An amorphous dielectric film of tantalum oxide is grown anodically on tantalum and the stability and dielectric properties of this film result in the high performance of tantalum capacitors. Tantalum capacitors are used in applications requiring small form factors and high reliability across a broad range of operating voltages and temperatures. Tantalum capacitors are used in mission-critical application such as military, aerospace and medical.
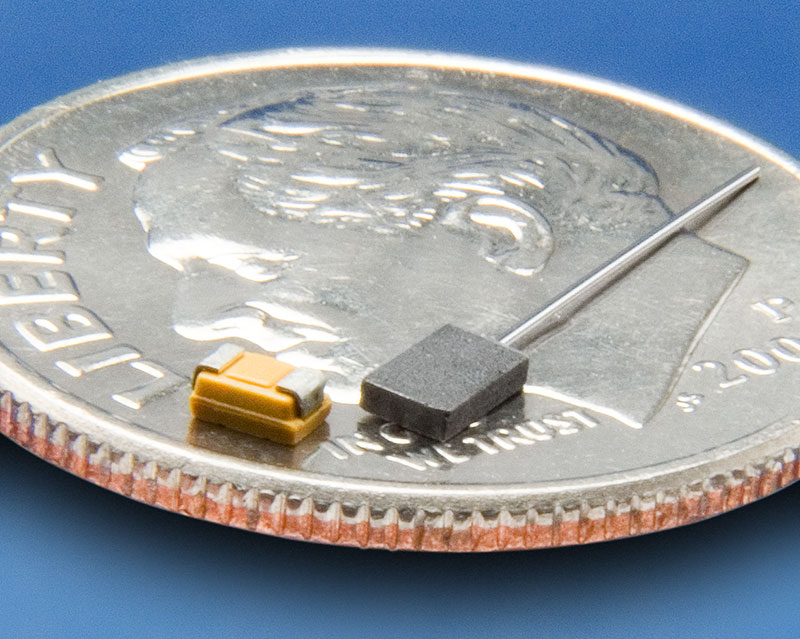
Tantalum capacitor sintered anode with tantalum wire (right) and fully bonded surface mount capacitor (left)
Tantalum has a variety of applications based on its unique corrosion resistance and mechanical properties4,5. For example, it can be worked into tube, thermowells, heat exchangers, plate, wire, and high temperature reactors. Tantalum serves these applications in industries that demand low contamination and severe conditions such as elevated temperature, strong acid environments, and other corrosive chemicals such as organics6,7. Use of tantalum also allows for the increase in process temperatures which can improve efficiencies in some processes.
Tantalum can also be coated onto base metal parts to provide the same corrosion resistance as a pure tantalum parts with lower cost, but with a shorter life span. Thermal spray processes alloys deposited tantalum powder to the surface of stainless steel parts in order to provide a tantalum coating that will not shear away under 180° bending tests8,9. Another method to coat parts with tantalum is cold spray. In this process, tantalum powder is accelerated supersonically on to a substrate. The momentum transfer during impact, deform and bond the tantalum particles to the base substrate without addition of external heating. This allows for cost effective use of tantalum while still retaining the corrosion resistant benefits. Methods to explosion bond tantalum onto reactor wall are routinely used in the chemical processing industry (CPI). Other coatings applications have been used in medical devices as well.
Tantalum can be machined using standard fabrication techniques such as turning, milling and grinding. Also, tantalum can be easily punched or stamped with steel dies10. Tantalum parts can be machined with a lathe using cemented carbide tools and high cutting speed to produce satisfactory results. These parts can be cleaned using hot hydrochloric acid or chromic acid to remove impurities from processes such a grit blasting or other machining. This refractory metal can be welded to other tantalum components or certain other alloys and metals. For welding tantalum to itself, inert gas arc welding is an effective method. Resistive welding can be used to achieve good welds between tantalum and other metals. Tantalum is oxygen sensitive at high temperature, so acetylene is not a viable option for tantalum welding. In addition to fabrication of tantalum components via traditional manufacturing techniques, advanced manufacturing methods like Additive Manufacturing, AM, (3D Printing) of tantalum are being developed. AM processes have enabled the production of highly complex tantalum parts for applications ranging in medical11,12, aerospace, automotive and defense markets.
Tantalum is an ideal barrier metal for use in integrated circuits, providing low contact resistance, good coverage, and blocking copper from diffusing into the silicon substrate13,14,15. Tantalum (or tantalum nitride, TaN) is deposited via PVD sputtering using a target. While other materials can be used as a barrier layer, tantalum adheres well to both copper and silicon while being inert to both processing and use temperatures.

Tantalum’ s unique physical and chemical properties give it the ability to support a broad range of markets and applications. These same properties allow tantalum to provide superior solutions where materials that have good corrosion and temperature resistance, workability, and ductility are required.
1 Sears, G. W. School Science and Mathematics 1918, 18 (2), 145–151.mju
2 Hampel, C. A. Corrosion Journal of Science and Engineering 1958, 29–32.
3 Titanium technical data http://www.thyssenkruppaerospace.com/materials/titanium/titanium-technical-data.html (accessed May 24, 2018).
4 Metal Selection for severe corrosion application https://tantaline.com/DOCs/Technical-
5 Taylor , D. F. “Acid Corrosion Resistance of Tantalum, Columbium, Zirconium, and Titanium”. Industrial & Engineering Chemistry 1950 42 (4), 639-639.
6 Cox, F. G. Anti-Corrosion Methods and Materials 1960, 7 (3), 69–74.
7 E.O.Ezugwu, “Key improvements in the machining of difficult-to-cut aerospace superalloys,” International Journal of Machine Tools and Manufacture, 2005, 45, 12–13, pp. 1353-1367.
8 Buckman, R. W., “New applications for tantalum and tantalum alloys,” JOM 2000, 52 (3), 40–41
9 T. Van Steenkiste, Gorkiewicz D. W. , “Analysis of tantalum coatings produced by the kinetic spray process,” Journal of Thermal Spray Tech., 2004, 13, 2, pp. 265–273.
10 Tantalum Machining http://www.espimetals.com/index.php/technical-data/242-technical-data/tantalum-machining/250-tantalum-machining (accessed Jun 19, 2018).
11 Levine BR, Sporer S, Poggie RA, Valle CJD, Jacobs JJ. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials. 2006; 27:4671–4681.
12 R. Wauthle, J. der Stok, S. A. Yavarid, J. V. Humbeeck, J.P. Kruth, A. A. Zadpoor, H. Weinans, M. Mulier, J. Schrooten, “Additively manufactured porous tantalum implants”, Acta Biomaterialia, 14 (1) March 2015, Pages 217-225).
13 H. Xiao, Introduction to Semiconductor Manufacturing Technology, 2nd ed., 2012.
14 Holloway, K.; Fryer, P. M.; Cabral, C.; Harper, J. M. E.; Bailey, P. J.; Kelleher, K. H. Journal of Applied Physics 1992, 71 (11), 5433–5444.
15 Laurila, T. Tantalum-based diffusion barriers for copper metallization. thesis, 2001.
Citing third party research
For more information contact:
Gordon Smith
Chief Technology Officer
e: GCSmith@globaladvancedmetals.com
1223 County Line Road, Boyertown, PA, 19512, US
Ryan Clement
Chief Commercial Officer
e: RClement@globaladvancedmetals.com
100 Worcester Street, Suite 200, Wellesley, MA, 02481, US
DISCLAIMER: The information contained in this document was obtained from third party sources. GAM has not independently tested or confirmed the accuracy or conclusions of these sources and GAM is not responsible for any errors or omissions, or for the results obtained from the use of the information provided in this article and the articles cited below. All information in this article is provided “as is”, with no guarantee of completeness or accuracy of the results obtained from the use of the information in this article and the articles cited below, and without any kind of warranty, express or implied.
